The degree of unsaturation relates the molecular formula of the compound to available possible structures. Individuals now are accustomed to using the net in gadgets to see image and video information for inspiration and according to the name of this article I will discuss about Degree Of Unsaturation Formula.

Degrees Of Unsaturation Or Ihd Index Of Hydrogen Deficiency
Of unsaturation 2 2 X C N - H - halogens 2 page 5 of 20.

Degree of unsaturation formula. Where n i is the number of atoms with valence v i. In other words both the formula C 8 H 6 F 3 NO 2 and the formula C 8 H 8 have identical numbers of degrees of unsaturation. Degree of unsaturation Total number of rings Total number of double bonds 2 x total number of triple bonds The formula for degree of unsaturation is articulated like this if the molecular formula is given.
Determining degrees of unsaturation from a formula. From the degree of unsaturation the number of multiple bonds and. The most useful and first step that helps us to identify an unknown organic.
Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated. Degree of unsaturation formula Indeed recently has been hunted by consumers around us maybe one of you personally. Degree of Unsaturation 2 2 x Carbons Nitrogens - Hydrogens - Halogens2.
Hydrocarbons that contain two double bonds 2 rings or an alkyne a triple bond are said to contain two degrees of unsaturation and have the formula C n H 2n-2 representing a loss of an additional two hydrogens. The formula for degree of unsaturation is. Uses of the degree of unsaturation formula.
That is an atom that has a valence of x contributes a total of x 2 to the degree of unsaturation. The degree of unsaturation designates the number of π bonds or rings that the compound is comprised of. The degree of unsaturation tells you how may rings and multiple bonds are present in a compound provided you know the molecular formula of the compound.
The formula subtracts the number of Xs because a halogen X replaces a hydrogen in a compound. Example of the degree of. The molecular formula is abridged to CnHm and the degree of unsaturation formula is given by.
Hence the DoB formula divides by 2. Knowing the degrees of unsaturation make it easier to figure out the molecular structure and also helps when double-checking the number of pi bonds andor cyclic rings. Any compound C3H6 must have one degree of unsaturation because 2 2x3-621.
As such we know that this formula must correspond to a compound either one double bond or one ring as in propene and cyclopropene shown previously. The molecule contains one nitrogen so you subtract one hydrogen from the molecular formula. For a compound with formula C a H b N c O d X e where X is F Cl Br or I the degree of unsaturation is given by.
The degree of unsaturation formula is also known as index of hydrogen deficiency HD and it is a easy way to calculate the number of multiple bonds or rings in a unknown chemical structure. D e g r e e s o f u n s a t u r a t i o n 2 C 2 N H X 2 Here C. One degree of unsaturation is equivalent to 1 ring or 1 double bond 1 π bond.
Degree of Unsaturation General formula of the degree of unsaturation. Two degrees of unsaturation is equivalent to 2 double bonds 1 ring and 1 double bond 2 rings or 1 triple bond 2 π. The two oxygens in the molecule you ignore.
Degree of unsaturation 12 2 2a - b c - e To calculate the degree of unsaturation enter the values of a b c d and e and press calculate. This organic chemistry video tutorial explains how to calculate the degree of unsaturation or the index of hydrogen deficiency of a molecule given its molecu. Isomer -- molecular formula dictates the degree of unsaturation according to the formula below.
The general formula of alkanes CnH2n2 is used as the standard for determining and comparing different degrees of unsaturation as alkanes are saturated and the simplest in composition among organic molecules. This gives a reduced equation of C 8 H 631 C 8 H 8. The Degree of Unsaturation is defined as the sum of the number of the rings involved plus the total number of multiple bonds present in an organic compound.
The result is then halved and increased by 1. For hydrocarbons that are compounds comprising of only hydrogen and carbon the degree of unsaturation formula is.
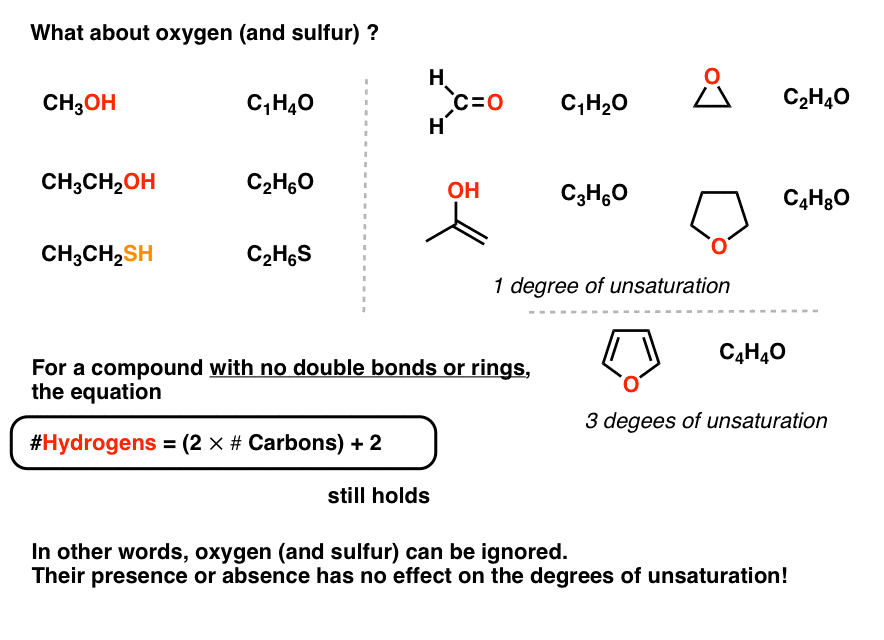
Degrees Of Unsaturation Or Ihd Index Of Hydrogen Deficiency

Degree Of Unsaturation Index Of Hydroden Deficiency Ihd Youtube

Degree Of Unsaturation Wikipedia
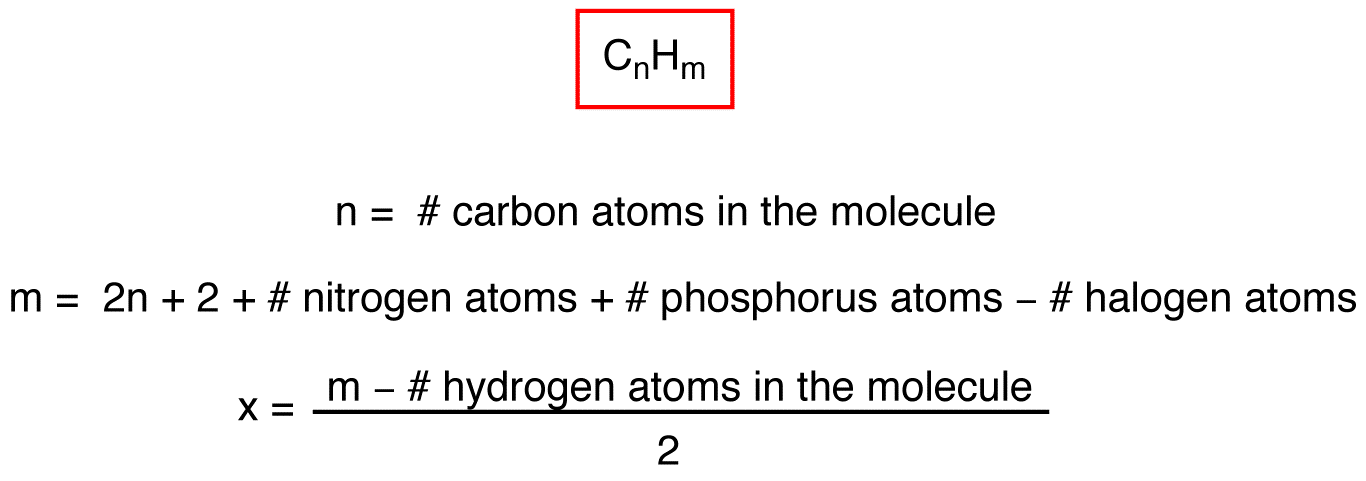
Degree Of Unsaturation Chemistry Libretexts

Degree Of Unsaturation With Halogens Oxygen And Nitrogen Youtube