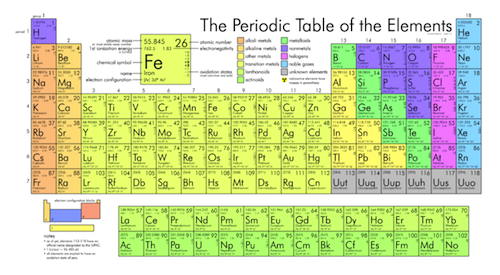
Element Family Element families are elements that have the same number of valence electrons. This product includes eight mini - posters one for each of the element families on the main group of the periodic table.

Periodic Table Element Families Diagram Quizlet
Each family has a specific name to differentiate it from the other families in the periodic table.
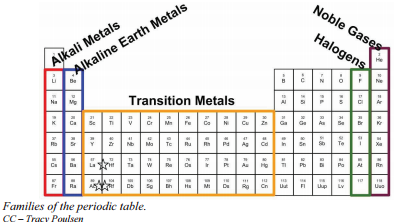
Families on the periodic table. Families on the Periodic Table. The IA family is made up of the alkali metals. The f-block columns between groups 3 and 4 are not numbered.
Groups usually have more significant periodic trends than periods and blocks explained below. Another common method of categorization recognizes nine element families. Most element families are a single column of the periodic table although the transition elements consist of several columns plus the elements located below the main body of the table.
Groups are the elements having the same outer electron arrangementThe outer electrons are also called the valence electrons. Group 1A of the periodic table. Aug 4 2017 - Students will be coloring the different families of the Periodic Table.
Groups 3 to 12 of the periodic table. Groups 3-12 - d and f block metals have 2 valence electrons Boron Group or Earth Metals. Elements within the same group have the same number of valence electrons.
Group 1 IA - 1 valence electron Alkaline Earth Metals. Group 2 IIA - 2 valence electrons Transition Metals. An example of an element family is the nitrogen group or pnictogens.
In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elements. Elements on the periodic table can be grouped into families bases on their chemical properties. Halogens form ionic bonds with all kinds of elements.
The figure below lists some important families that are given special names. Each family has a. Group 7A of the periodic table.
To differentiate it from the other families in the periodic table. A vertical column in the periodic table. The completed colored chart can be used as a resource when playing the interactive games created by CHEM by Gloria.
Elements on the periodic table can be grouped into families bases on their chemical properties. This similar electron configuration leads to the elements in the same group having similar chemical properties. Alkali Metals Alkaline Earth Metals BoronAluminum Group Icosagens Carbon Group Crystallogens Nitrogen Group Pnictogens Oxygen Group Chalcogens Halogens and Noble Gases.
Are located in the second column from the right side of the periodic table group 17. Alkali metals Group 1 IA 1 valence electron Alkaline earth metals Group 2 IIA 2 valence electrons Transition metals Groups 3-12 d and f metal blocks have 2 valence electrons Boron group or earth metals. Elements in each family react differently or the same with other elements.
The Periodic table can be divided into nine families of elements each having similar properties. In reactions these elements all tend to lose a single electron. The halogen family consists of fluorine chlorine bromine iodine and astatine.
Another common method of categorization recognizes nine families of elements. What does each family have to differentiate it from the other families in the periodic table. Group 1 of the periodic table are the alkali metals.
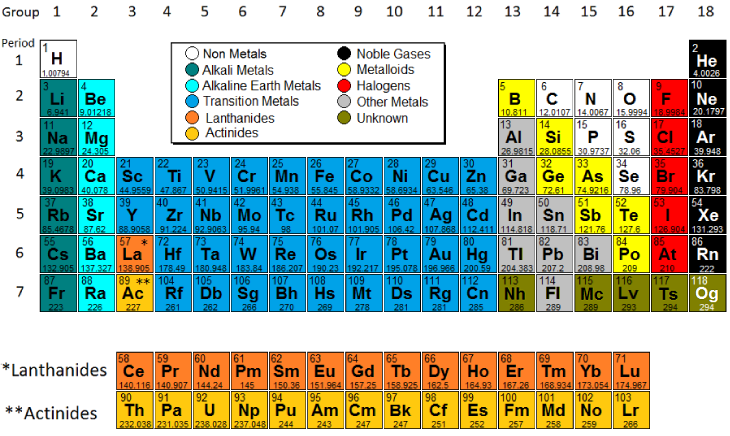
There are 18 numbered groups in the periodic table. Group 1 or the first column on the periodic table are called ____. They all have seven valence electrons which make them highly reactive for covalentbonds.
The group family number is the number assigned to the vertical columns of the periodic table. Elements in each family. A group is a vertical column in the Periodic Table of Elements.
When the elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. They are highly reactive and do not occur freely in nature. Group 2A of the periodic table.
Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number. Group 8A of the periodic table. The IIA family is made up of the alkaline earth metals.
All these elements tend to lose two electrons. They are considered the most important method in classifying the different elements. A group or family is a vertical column in the periodic table.
Periodic Table Model Science Software
:max_bytes(150000):strip_icc()/Elements-58f7944e5f9b581d59396ae7.jpg)
Element Families Of The Periodic Table
The Periodic Table Families The Periodic Table