Mixing two pure substances results in a mixture. It has the same properties and the same ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory.
/definition-of-pure-substance-605566_FINAL-d1c54ff9183944028aa8e213936affdf.png)
Pure Substance Definition And Examples
Also a pure substance can be defined as any single type of material that has not been contaminated by another substance.
Example of a pure substance. Put your understanding of this concept to test by. To avoid confusion a pure substance is often referred to as a chemical substance Examples of Pure Substances. Chemists can classify matter as solid liquid or gas.
A pure substance participates in a chemical reaction to form predictable products. Pure substances are made of only one type of atom or molecule. Examples of Pure Substances.
If salt were added to the beaker then a mixture is produced. If true then enter 1 else enter 0. Gold pure water hydrogen gas.
All of the above. Is used in chemistry in a different way from its everyday meaning. Examples of pure substances include chemical elements and compounds.
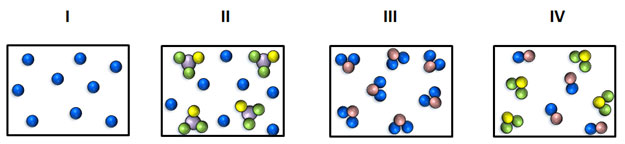
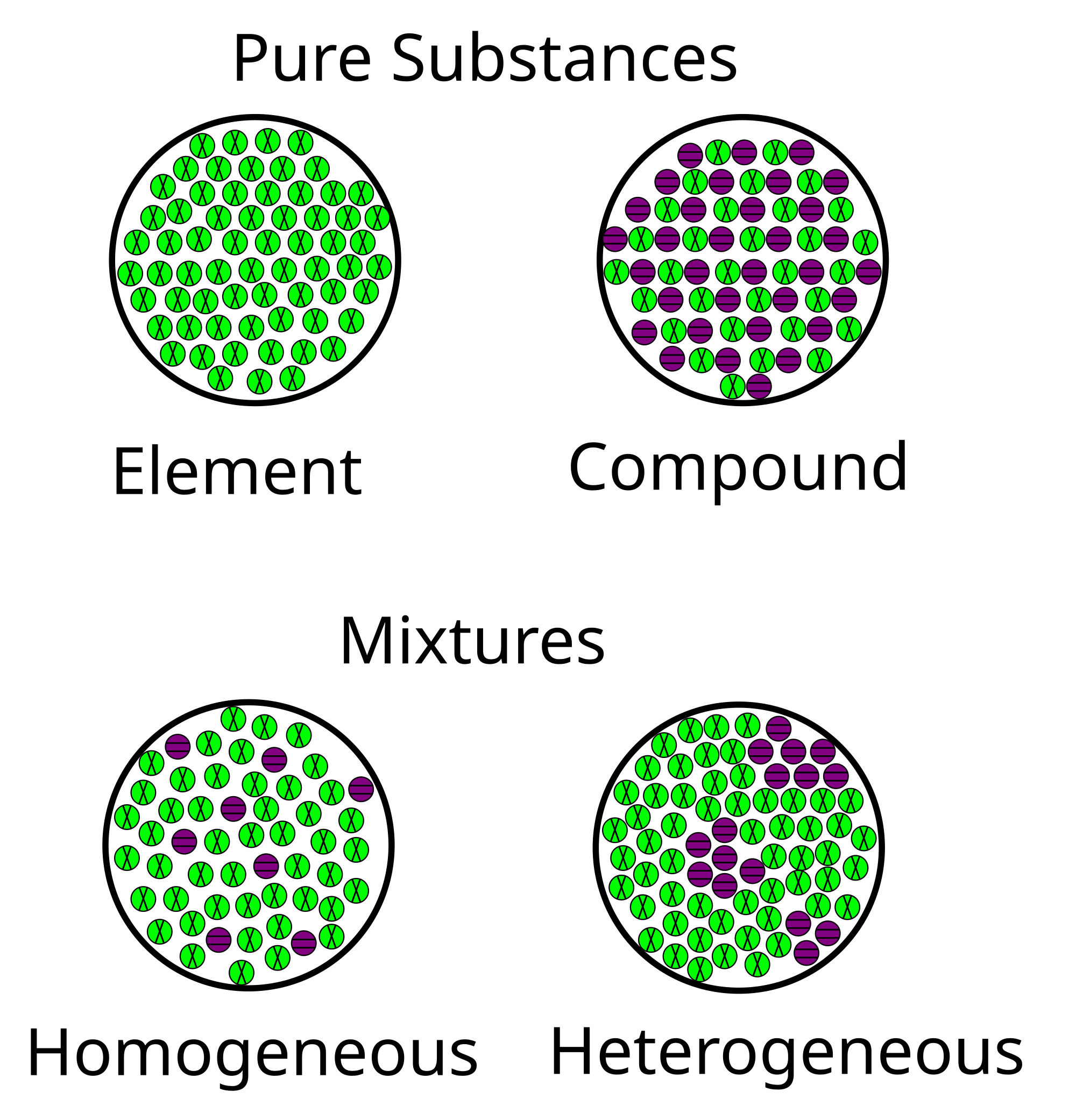
A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together Distinguishing between pure substances and mixtures. A mixture consists of two or more elements or compounds that are physically mixed together they are not chemically combined. Complete the statement - an element is a pure substance made up of identicaldifferent atoms.
A mixture on the other hand is the physical combination of two or more substances. Homogeneous and Heterogeneous Mixture. A pure substance usually participates in a chemical reaction to form predictable products.
Pure substances and mixtures The meaning of pure. In chemistry a pure substance is a material with a constant composition. Examples of pure substances include water gases like carbon dioxide oxygen and metals like platinum gold and silver.
Alloys and other solutions may also be considered pure if they have a constant. For example shops sell cartons labelled as pure orange juice. In other words it is homogeneous no matter when you sample it.
It can be a compound or a single element. For example a beaker of pure sample of water contains only H 2 O molecules and nothing else. The classification of matter.
In this article we are going to focus on pure substances. Pure substances A. A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample.
In chemistry a pure substance consists of only one type of atom molecule or compound. Compounds such as water salt or crystals baking soda amongst others are also grouped as pure substances. However in practice no substance is entirely pure and chemical purity.
- Hydrogen Oxygen Nitrogen Chlorine Bromine Iodine Carbon Silver Gold. For example shops sell cartons labelled as pure orange juice. Cloud is a colloidal suspension of water droplets in air.
Its a substance made of only one type of atom or only one type of molecule a cluster of atoms. Which of the following is a. In chemistry a pure substance has a definite composition.
In chemistry a pure substance may consist of a single element or compound which contains no other substance. Which of the following is an example of pure substance. All matter can be classified as either a pure substance or a mixture.
A pure substance consists entirely of one type of atom or compound. A pure substance refers to an element or a compound that has no component of another compound or element. But there are other ways to classify matter as well such as pure substances and mixtures.
Hydrogen gas and pure iron are examples of pure substances. Mixtures are physical combinations of two or more elements andor compounds. A few of them include gold copper oxygen chlorine diamond etc.
Examples of Pure. Test your Knowledge on Difference Between Pure Substance And Mixture. All elements are mostly pure substances.
A pure substance is any single type of material that are made of only one type of atom or only one type of molecule. A pure substance is made up of only one type of particle and has a fixed structure. To separate the two scientists use a.
In chemistry the pure substance is a very simple concept to grasp. Compounds are substances that are made up of more than one type of atom. Each pure substance has its own set of unique chemical and physical properties which helps us in identifying.
Elements and compounds are both examples of pure substances. A pure substance or chemical substance is a material that has a constant composition is homogeneous and has consistent properties throughout the sample. Examples of Pure and Impure substancesAnswerExamples of a pure substance -Elements.
Examples include water liquid diamond solid and oxygen gas. The word pure is used in chemistry in a different way from its everyday meaning. Hydrogen consists of hydrogen atoms only while iron consists of only iron atoms.
A pure substance is a type of matter which exists in its most basic or purest form and cannot be broken down further. It participates predictably in a chemical reaction. To learn more about the difference between pure substances and mixtures register with BYJUS and download our app.
In chemistry a pure substance is a sample of matter with both definite and constant composition and distinct chemical properties. A common example of a chemical substance is pure water. Other chemical substances commonly encountered in pure form are diamond carbon gold table salt sodium chloride and refined sugar.
Pure substances The meaning of pure. Mixtures can be classified as homogeneous or heterogeneous. Oil water sand sugar.
Classification is one of the basic processes in science. An element is a pure substance that cannot be separated.

Classifying Matter Mixtures And Pure Substances Lessons Blendspace

Pure Substances And Mixtures Elements Compounds Classification Of Matter Chemistry Examples Youtube

Ncert Class 9 Science Solutions Chapter 2 Is Matter Around Us Pure Part 1 Flexiprep